In clinical blood group antibody screening and identification, recipients, who have a history of blood transfusions or pregnancy, as well as donors, should all undergo incomplete antibody testing. Incomplete antibody detection is one of the most important elements for a clinical diagnosis of several diseases, including hemolytic disease in newborns, autoimmune hemolytic disease, drug immune hemolytic diseases, and so on.
Traditional tube coombs test (Tube-Coombs T) need several washing steps which are required to remove unbound globulins, but they may cause false negatives. Microcolumn gel immunoassay Coombs test (MGIA-Coombs T) overcomes the tedious washing steps and becomes the regular method in the detection of incomplete antibodies. One disadvantage of the MGIA assay is some fibrin, particulates or other artifacts may trap red blood cells at the top of the gel columns erroneously leading to an abnormal result. In addition, the expense of the MGIA assay is cost prohibitive for mainstream clinical use.
Here, Suzhou Institute of Biomedical Engineering and Technology Chinese Academy of Sciences (SIBET) presents an improved approach employing hydrogel chromatography medium (HCM) in the detection of incomplete antibodies. After a rapid single-step centrifugation, incomplete antibodies, attached to red blood cells (RBCs), were separated from the reaction mixture using HCM and sedimentation. This method obviates the need for multiple centrifugation steps found in conventional Tube-Coombs tests. The HCM-Coombs tests may have a wide range of applications for incomplete antibody detection.
The relevant research achievements have been published in the Transfusion and Apheresis Science, 2015, 53(3): 337-341(SCI,IF=0.768)
Paper links: http://www.trasci.com/article/S1473-0502%2815%2900133-0/abstract
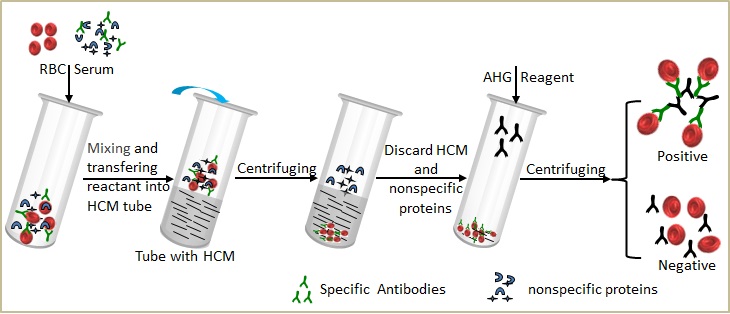
Fig. 1Flow chart of experimental procedure for incomplete antibody detection using HCM
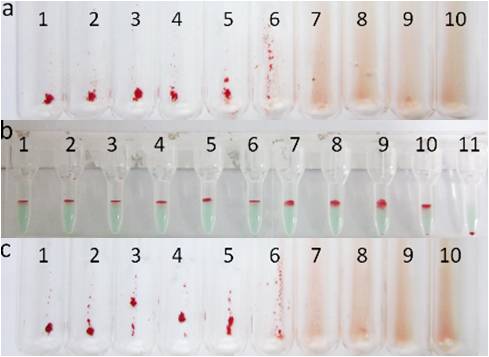
Fig. 2 Agglutination of different anti-D dilutions in three different methods. (a) Tube-Coombs T, (b) MGIA-Coombs T, (c) HCM-Coombs T, 1–10: 1×, 2×, 4×, 8×, 16×, 32×, 64×, 128×, 256×, 512×, 11: negative control.
(Information source: Nanjing branch of CAS)

