Pentachlorophenol (PCP) is a weak organic acid, which can be totally or partially ionized in normal water and sediments, affecting its sorption, transport and bioavailability in lakes. PCP, heavy metals and phosphate coexist in some eutrophic lakes with cyanobacteria. PCP in water column could adsorb on living and nonliving cyanobacteria during the algal blooms, which may be affected by the coexisting heavy metals or phosphates. Once adsorbs on cyanobacteria, PCP can be transferred to bottom water and sediment with the settling or dead cells or ingested by higher organisms, resulting in more extensive contamination in eutrophic lakes. However, to date little is known about the influences of pH, heavy metals and phosphate and their co-influences on the sorption of PCP on biomass of cyanobacteria.
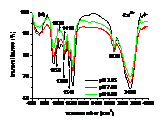
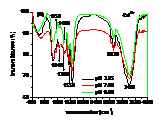
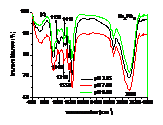
Influences of pH, heavy metals (Cu2+, Cd2+) and phosphate (Na3PO4) and their co-influences on the sorption of PCP on the biomass of cyanobacteria derived from a natural bloom were thereby investigated. Results indicated that sorption of PCP significantly decreases with pH in the range of 3.25 to 9.00. Although sorption coefficient of ionized PCP was 8.51 times lower than that of neutral species, it was the dominant species at environmentally relavent pH and contributed more to the total sorption of PCP. In the presence of low concentration of Cu2+ (≤ 40 μmol L-1), sorption of PCP was much lower than that of the blank. However, it increased gradually with Cu2+, and overpassed the blank when concentration of Cu2+ was higher than 50 μmol L-1. Compared with the sole influence of pH, coexisted Cu2+ inhibited the sorption of PCP at pH of 3.25 and 4.35, but enhanced it in the pH range of 5.00 to 9.00. In the presence of Cd2+, sorption of PCP first increased then decreased rapidly and finally increased slightly again with the increase of Cd2+. Except for at pH of 9.00, sorption of PCP at other pH in the presence of Cd2+ was much lower than that solely affected by pH. In the presence of Na3PO4, sorption of PCP increased rapidly then maintained with the increase of Na3PO4. In the presence of both Na3PO4 and pH, sorption of PCP at pH from 3.25 to 5.00 was lower than that solely affected by pH, while it increased with pH in the range of 5.00 to 9.00 and was higher than that solely affected by pH in the range of 6.00 to 9.00. Ion pairs of pentachlorophenolate-metal facilitated the sorption of PCP, which was largely dependent on pH illustrated by UV-visible and FTIR spectra. Speciations of metals and PCP and the stability constants of ion pairs of pentachlorophenolate-metal greatly affected the sorption. Ionic strength also played an important role for the sorption of PCP.
This study highlights the influences of pH, different types of transition metals (Cu2+, Cd2+) and nutrient (Na3PO4) and their co-influences on the sorption of PCP on the biomass of cyanobacteria derived from natural blooms for the first time, which has important implications in understanding the sorption and biogeochemical cycles of PCP, and the potential interactions between different types of pollutions in eutrophic lakes. This work is currently published on Water Research (Yuqiang Tao*, Bin Xue, Jicheng Zhong, Shuchun Yao, Qinglong Wu. Influences of pH, heavy metals and phosphate and their co-influences on the sorption of pentachlorophenol on cyanobacterial biomass. Water Research, 2012, 46, 3585-3594). This work was funded byNational Basic Research of China (2008CB418104), Natural Science Foundation of China and Jiangsu Province (21107118 and BK2010604)and President Scholarship Foundation of Chinese Academy of Sciences.

(Information source: Nanjing Institute of Geography & Limnology of CAS)

