Persulfate (PS)-based in-situ chemical oxidation (ISCO) for the remediation of contaminated soils and groundwater has attracted great attention in recent years. Diverse contaminants including chlorinated compounds, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and refractory pollutants can be degraded by PS activated with heat, UV light, bases, H2O2, and transition metals; the efficiency of each treatment depends on the contaminant type and subsurface conditions. The soil or subsurface components such as naturally occurring minerals and organic matter affect the activation of PS and formation of reactive oxygen species (ROS) that influence the remediation efficiency of contaminated soil. Therefore, the effects of subsurface chemistry on PS activation have been widely investigated. For example, it has been reported that quinones or phenols activate PS efficiently for contaminant degradation. Minerals such as magnetite, pyrite, and siderite can also activate PS. Thus, naturally occurring minerals play an important role in PS decomposition and contaminant degradation. Most of the previous studies mainly investigated the effects of Fe and Mn-minerals on PS activation and contaminant degradation; however, the activation of PS with other minerals such as vanadium species has been rarely investigated.
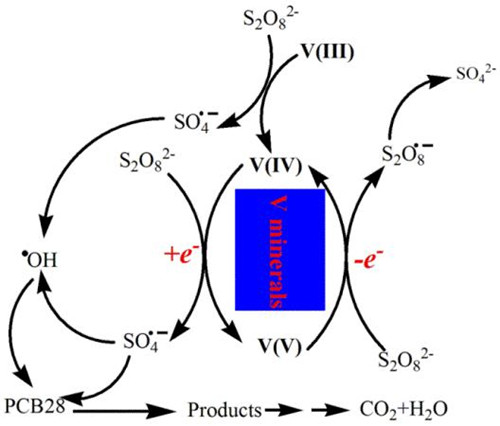
Therefore, the main objective of this study was to explore the activation of PS with V species for degradation of organic contaminants. In the present study, the activation of persulfate (PS) with vanadium (V) species for PCBs (2,4,4′-trichlorobiphenyl [PCB28]) degradation was investigated for the first time. It was found that V2O3 exhibited high catalytic activity toward PS decomposition for PCB28 degradation. Even under near neutral pH (7.4), PCB28 was efficiently degraded in V2O3/PS. Sulfate radical anions (SO4 −) and hydroxyl radicals (
−) and hydroxyl radicals ( OH) were produced from PS activation with V2O3 for PCB28 degradation, and were characterized with electron paramagnetic resonance (EPR) technique. Free radical quenching studies showed that ethanol inhibited PCB28 degradation, while tert-butyl alcohol enhanced PCB28 degradation via reductive dechlorination with alcohol radicals. The pathway of PCB28 degradation was proposed on the basis of GC–MS analysis of intermediates of PCB28 degradation in V2O3/PS. The mechanisms of PS activation are elucidated. It was found that V(III) in V2O3 activated PS to form SO4
OH) were produced from PS activation with V2O3 for PCB28 degradation, and were characterized with electron paramagnetic resonance (EPR) technique. Free radical quenching studies showed that ethanol inhibited PCB28 degradation, while tert-butyl alcohol enhanced PCB28 degradation via reductive dechlorination with alcohol radicals. The pathway of PCB28 degradation was proposed on the basis of GC–MS analysis of intermediates of PCB28 degradation in V2O3/PS. The mechanisms of PS activation are elucidated. It was found that V(III) in V2O3 activated PS to form SO4 − and V(IV) (VO2) via electron transfer process, and the formed V(IV) further transferred an electron to PS to generate SO4
− and V(IV) (VO2) via electron transfer process, and the formed V(IV) further transferred an electron to PS to generate SO4 −and V(V) (V6O13), which were supported with XRD analysis. Furthermore, both VO2 and V2O5 can activate PS for PCB28 degradation, indicating that V(IV) would be regenerated from the reduction of V(V) by persulfate ions (S2O82−) on the surface of V2O5 particles. These findings would help to better understand the interactions between naturally occurring V minerals and PS, and provide a novel activator for PS activation to degrade contaminants.
−and V(V) (V6O13), which were supported with XRD analysis. Furthermore, both VO2 and V2O5 can activate PS for PCB28 degradation, indicating that V(IV) would be regenerated from the reduction of V(V) by persulfate ions (S2O82−) on the surface of V2O5 particles. These findings would help to better understand the interactions between naturally occurring V minerals and PS, and provide a novel activator for PS activation to degrade contaminants.
The research achievements have been published in the Applied Catalysis B: Environmental (IF 8.328), 2017,202, 1-11, Activation of persulfate with vanadium species for PCBs degradation: A mechanistic study.
Paper links: http://www.sciencedirect.com/science/article/pii/S0926337316306853
(Information source: Nanjing branch of CAS)

