Oxygen, takes 21% volume of the atmosphere and composes 48.6 weight of the earthcrust, also accomplishes the species diversity on the earth, due to its chemical activity and electronegativity. Since the initial discovery by AL Laroisier in 1777, oxygen has drawn great attentions from chemists of generations, and now it is shining again in the emerging field of semiconductor SERS.
Surface Enhanced Raman Scattering (SERS) has distinguished itself from normal Raman by the introduction of substrate materials. On the surface of SERS substrates, the cross section of Raman scattering from analyte molecules can be greatly enlarged, leading to significant magnification of Raman signals. Since the discovery of surface enhanced Raman scattering (SERS) in the 1970’s, the sensitivity of Raman detection has been improved by over a million-fold benefiting from the introduction of noble-metal substrates. Conquering the inherent disadvantage of weak signals tangling with normal Raman measurement, SERS detection has gained wide application in the fields varying from food safety, environmental monitoring to life science, and soon became one of the most sensitive in-situ spectral detection techniques for surface species. Unfortunately, SERS experiments have been essentially dominated by adsorbates on rough metallic surfaces, especially limited to noble metals such as Au, Ag and Cu. In addition, noble metals typically show poor stability and biocompatibility in practical use, and therefore the search for other alternative materials as highly active SERS substrate becomes an urgent task. Nowadays, semiconducting compounds as SERS substrate have become appealing, for their luxuriant compositions and wide applications. However, the generally low SERS enhancement shown by semiconductors seems to be the bottleneck hard to break in the field of SERS research. As is well established, SERS enhancement arises from the interaction between analyte and the surface of substrate, contributed through two types of mechanisms: the first one, Electromagnetic mechanism (EM) that is usually found with metal substrates. The second one, chemical mechanism (CM) governs the enhancement of semiconductor substrates. Just because of the discrepancy in mechanism, the design of substrate with semiconductors should be totally different from that prevailed with noble metals in the past.
Recently, Professor ZHAO Zhigang’s group from Suzhou Institute of Nanotech and Nanobionics (SINANO), Chinese Academy of Sciences has succeeded in the finding that oxygen can be the key to unlock the activity of semiconductor as SERS substrates. They realized the enhancement of SERS signals from analyte on semiconductors that once were recognized as none- or weak-SERS active substrate, simply through the stoichiometry-modification of those transition metal compounds by lattice oxygen extraction or incorporation.
Firstly, SERS enhancement based on oxygen extraction from W18O49.Under the guidance of this strategy, they employed vacancy-containing W18O49 sea urchin-like nanoparticles as the substrate material to achieve greatly enhanced SERS effect for the first time on function-rich tungsten oxide material, and the enhancement was further improved by creating surface deficiencies via H2, Ar thermal-reduction, which gave a detection limit as low as 10-7 M and the maximum enhancement factor (EF) of 3.4×105, in the rank of the highest sensitivity among semiconducting materials, even comparable to noble metals without ‘hot spots’. The results have demonstrated that oxygen vacancy could be of crucial importance to the SERS activity of semiconducting oxides.
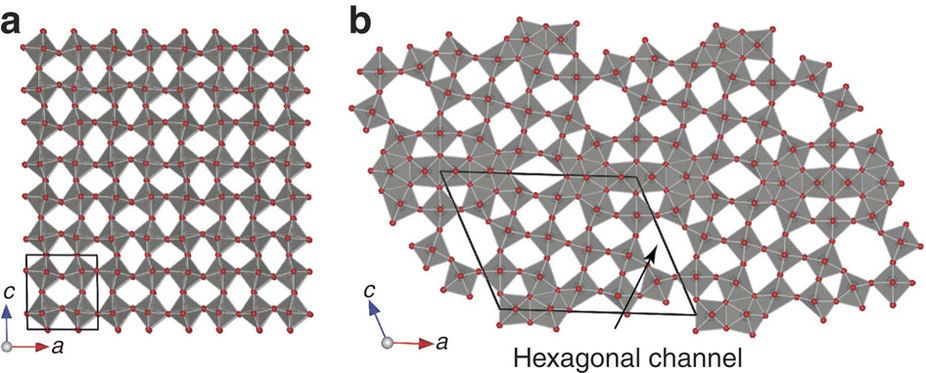
Figure 1. Crystal structure of (a) stoichiometric WO3 and oxygen-deficit W18O49.
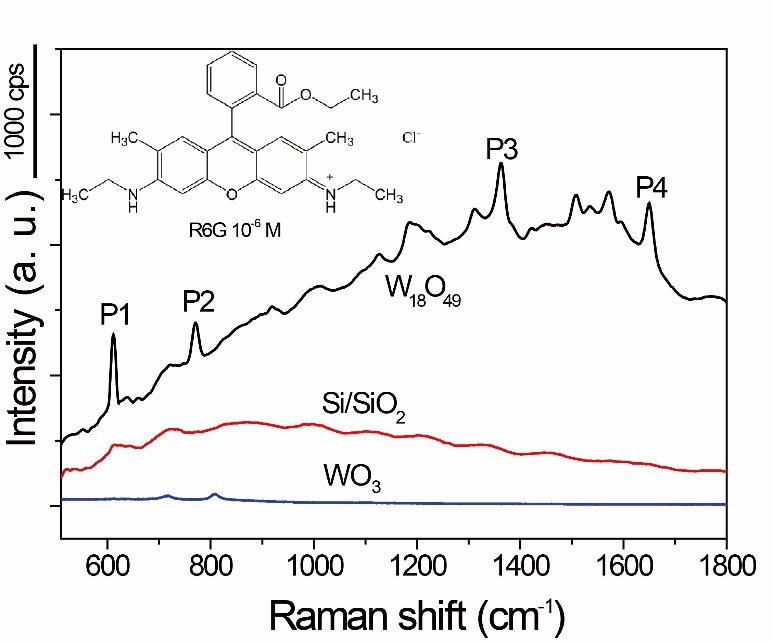
Figure 2. Oxygen-deficit W18O49 as excellent SERS substrate.
Secondly, SERS enhancement based on oxygen incorporation into MoS2 .Oxygen extraction from lattice has shown its effectiveness towards SERS-active semiconductors, while instead, what will happen to the materials with oxygen incorporation? At this time, Zhao’s group started with MoS2—a typical chalcogenide with rather weak SERS activity, in which the oxygen incorporation could be realized either during the low-temperature hydrothermal synthesis or through the post thermal treatment in air. It was evidenced that oxygen incorporation with proper amount could account for the one-million-fold magnified SERS enhancement in comparison with that of the pristine unincorporated MoS2, while further incorporated oxygen into the lattice led to a sharply degraded SERS performance. Additionally, such a strategy of oxygen incorporation has shown its universality in several other chalcogenide compounds as SERS substrates to achieve greatly improved performance, including WSe2, WS2, MoSe2, and etc.
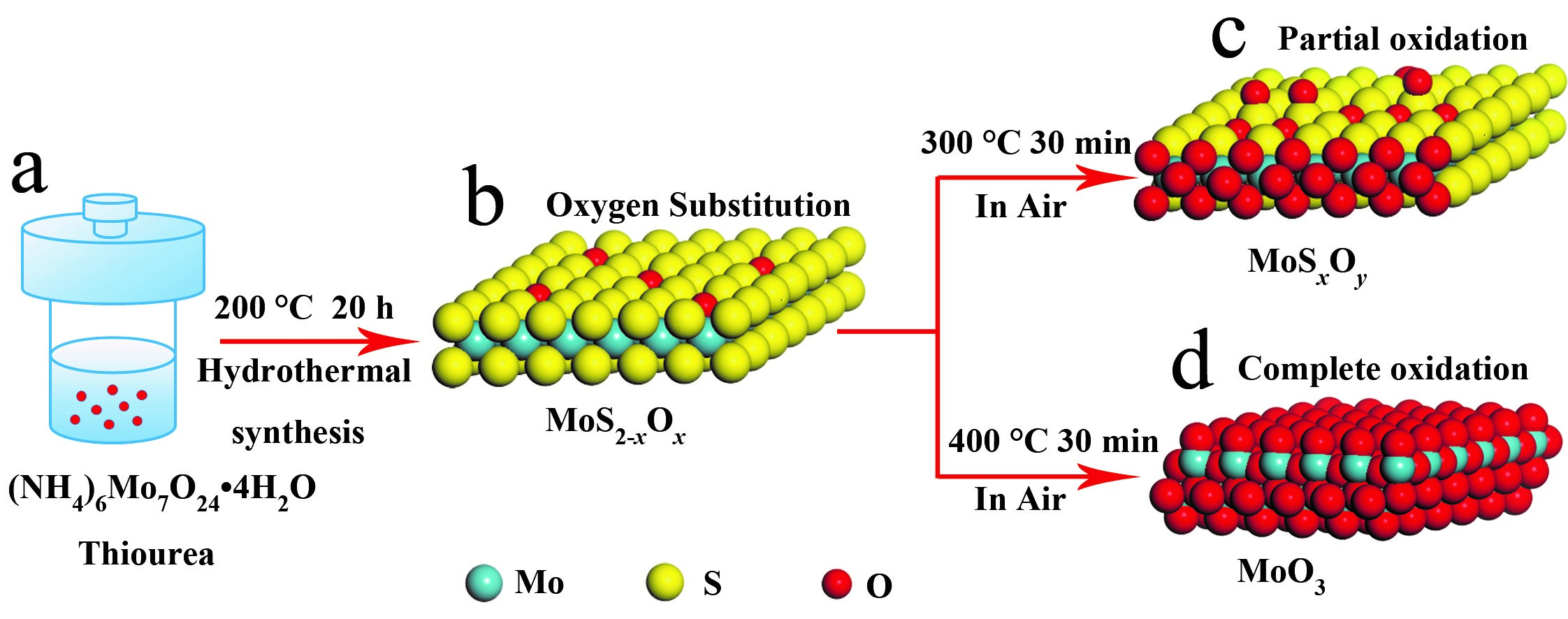
Figure 3. Oxygen incorporation into the lattice of MoS2.
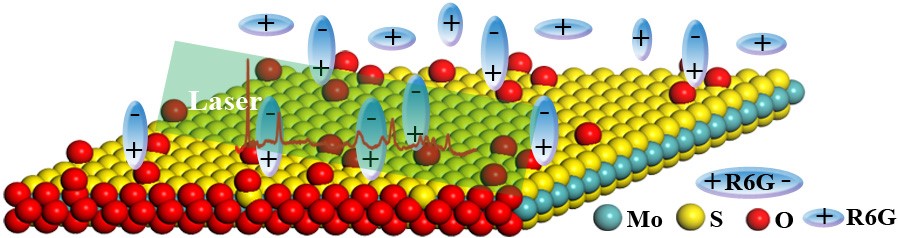
Figure 4. Scheme showing the SERS on MoS2 substrate after oxygen incorporation.
Thirdly, SERS enhancement mechanism in association with oxygen extraction/incorporation. Now, the SERS enhancement of semiconductors benefiting from either oxygen extraction or incorporation has already been unified, which is further supported by the results of theoretical calculations. Based on the chemical mechanism (CM) of semiconductor-molecule system, both adding and extracting of oxygen into/from the semiconductor lattice are all proved to be effective means in its band-structure modification. Deep levels in the bandgap introduced by “oxygen extraction” acts as an springboard for electron transfer, while the increased density of state near Fermi level after “oxygen incorporation” narrows the bandgap, both of which will facilitate the charge carrier generation in a laser-stimulated semiconductor, further contribute to the charge-transfer between substrate and analyte through vibronic coupling, then increase the polarization tension of adsorbed molecule, and finally give the increased Raman signals.
The systematic work reveals that under a suitable modulation of the lattice oxygen density in semiconductor oxides, the SERS activity of which can be dramatically promoted. These findings break through the limitation of noble metal substrates in common SERS applications, and provide important clues in the future design strategy for highly-efficient semiconducting SERS substrates.
This work was supported by the National Natural Science Foundation of China (51372266, 51572286, 21503266, 51772319 and 51772320), the Outstanding Youth Fund of Jiangsu Province (BK20160011), and the Youth Innovation Promotion Association, CAS. Relative results have been successively published in Nature Communications (on July 17, 2015 and December 8, 2017), with the title of “Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies”and“Semiconductor SERS enhancement enabled by oxygen incorporation”, respectively. (DOI: 10.1038/ncomms8800 & DOI: 10.1038/s41467-017-02166-z)
Contact information:
Prof. ZHAO Zhigang, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences
Email:zgzhao2011@sinano.ac.cn
Reference: https://www.nature.com/articles/ncomms8800
https://www.nature.com/articles/s41467-017-02166-z

