In cell biology studies, traditional high-throughput cell screening is performed using flow cytometers for automatically analyzing and sorting cells. Recently, imaging flow cytometers have been developed for imaging each cell in a flow. Then, the statistics of cell morphology can be deduced to differentiate cell types or to study the dynamics. A microfluidics-based flow cytometer has also been developed in recent years; this is in principle a microscopic imager of the microfluidic channel. Although all of the above instruments are useful for high-throughput cell imaging, they cannot be applied to miniature model organisms such as zebrafish because their embryos are hundreds of times larger than normal cells. Therefore, a new generation of instruments with much wider microfluidic channels as well as much larger imaging field of view is in great demand.
Recently, Prof. LI Hui’s group from Suzhou Institute of Biomedical Engineering and Technology (SIBET), Chinese Academy of Sciences (CAS), developed a high-speed zebrafish embryo imaging method using a flow imaging scheme.
In this smartly designed system, a fast linear charge-coupled device (CCD) was utilized to capture images of zebrafish embryos flowing through a capillary. Accordingly, a thin sheet of light was generated using a cylinder lens and illuminated on the samples to reduce the exposure of the living embryos to the laser. Although line-scan imaging has been widely applied in pathological slide scanning, and similar geometry was also used to image cells in microfluidic channel. It is the first time to realize living embryo imaging in a fluidic flow.
The following experimental results showed that up to 20 embryos could be imaged in just one second, which was 100 times faster than the traditional VAST system. They also developed algorithms for the automatic analysis of embryos with and without chorion. Dead and live embryos could be clearly distinguished by their gray level distribution, and this method could be further utilized to study the effect of temperature on the cumulative mortality rate. The analysis of the morphological characteristics of zebrafish embryos (i.e., their lengths and widths) was also demonstrated.
In conclusion, this linear-CCD-based flow imaging system (Lc-FIS) is very promising for large-scale studies of the development of miniature model organisms in terms of various factors and drug discovery.
This study was published in Biomedical Optical Express. It was supported by the Natural Science Foundation of China, the Natural Science Foundation of Jiangsu Province, scientific research equipment development project of the Chinese Academy of Sciences, and Hundred-Talent Program of the Chinese Academy of Sciences.
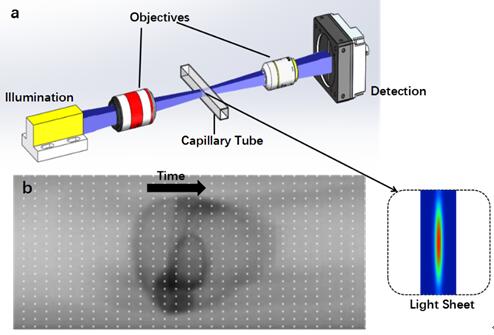
Fig 1. Principle of the linear-CCD-based flow imaging system (Lc-FIS). (Image by LI Hui)
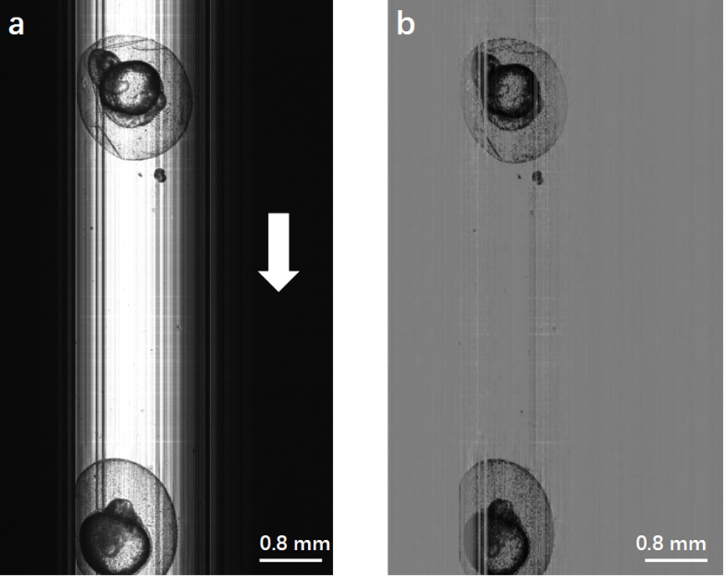
Fig 2. (a) Original image of zebrafish embryos obtained by Lc-FIS. (b) Preprocessed image after substracting the background. (Image by LI Hui)

